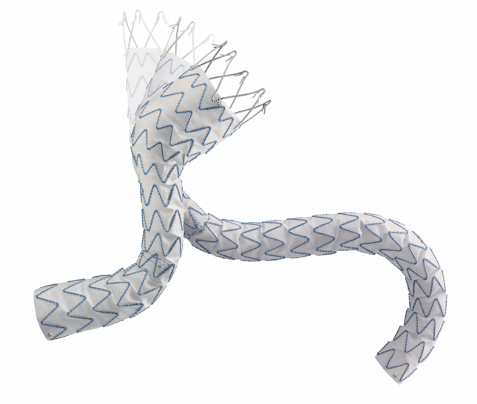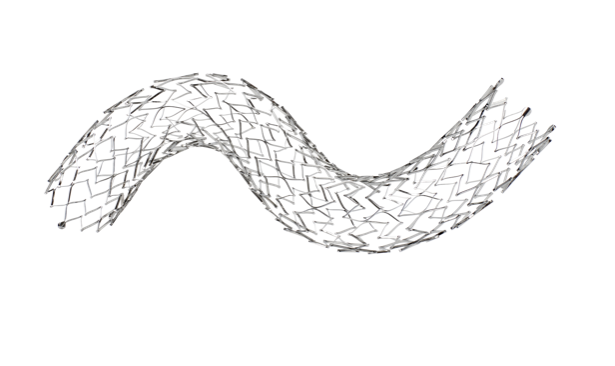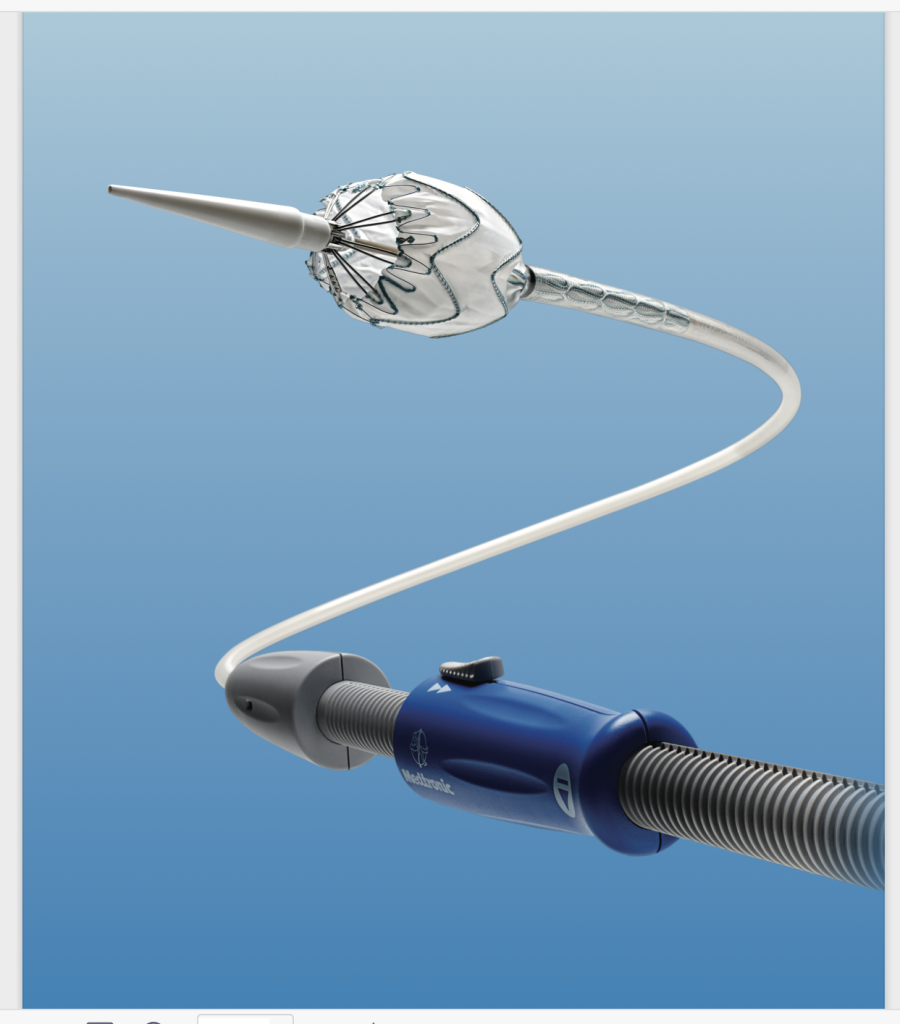
The Valiant stent graft is a modular, self-expanding device with nitinol scaffolding attached to the graft’s exterior. It’s preloaded in the Xcelerant Delivery System and offers longer components (up to 200 mm) compared to the Talent device, with a design that improves radial force distribution. At the proximal end, the Valiant features a bare-spring configuration (FreeFlo) with less flare than the Talent’s, which helps to evenly distribute the radial force, thereby reducing pressure on individual contact points. The distal end can be configured with either a closed web or an eight-peak design, providing flexibility based on clinical needs.